
Pharmaceutical Methods
Publishing Quality Research & Reviews

Pharmaceutical Methods
Publishing Quality Research & Reviews
Research Article - (2022) Volume 13, Issue 4
Received: Nov 01, 2022, Manuscript No. PHMETHODS-22-78811; Editor assigned: Nov 04, 2022, Pre QC No. PHMETHODS-22-78811 (PQ); Reviewed: Nov 18, 2022, QC No. PHMETHODS-22-78811; Revised: Nov 25, 2022, Manuscript No. PHMETHODS-22-78811 (R); Published: Dec 02, 2022, DOI: 10.35248/2229-4708.22.13.237
Introduction: In this research, a fit-for-purpose LC-MS/MS method for quantification of Imatinib Mesylate in rat plasma was developed and utilized for the pharmacokinetic study. Imatinib extraction was done and isolated from plasma samples using protein precipitation method. Imatinib quantification was done using Liquid Chromatography (LC) tandem Mass Spectrometry (MS) with Electro Spray Ionization (ESI) and Multiple Reaction Monitoring (MRM) in positive ionization mode.
Objective: A simple and fast fit for purpose LC-MS/ MS method was developed and utilized for the quantification of Imatinib mesylate and was applied to a pharmacokinetic study.
Methods: All the sample preparation was acquired and accepted through protein precipitation. High performance chromatographic partition was achieved on a PURITAS PNCN, (100 × 4.6 mm, 5 μm) CHROMACHEMIE analytical column by using an isocratic elution. Pump A (40%): 0.1% Formic acid in 5 mM Ammonium Formate Solution Pump B (60%): 0.1% Formic acid in acetonitrile at a flow rate of 0.8 mL/ min. for 5 mintues.
Results: The retention time of Imatinib mesylate and its internal standard, Verapamil was 3.21 ± 0.8 min and 3.64 ± 0.5 min, respectively. The total run time was 5.0 minutes. The elution detection was obtained with +ve electrospray ionization multiple reaction monitoring of the ion transitions at m/z 494.40 → 394.20 for Imatinib and second mass transitions were monitored: Imatinib at m/z 494.40 → 217.20 while internal standard Verapamil was selected for m/z 455.30 → 165.10. The method was developed and validated over the concentration range of 50- 5000 ng/mL for Imatinib mesylate, with correlation coefficient greater than 0.9991. The extraction recovery was more than 105.37% and the matrix effect was not significant. The intra and inter-day precisions were below 4.65% and accuracies ranged from 91.7 to 102.0%. The quantification limit for Imatinib was 5.05 ng/mL. Imatinib mesylate was demonstrated to be stable in rat plasma under the tested conditions.
Conclusion: The developed LC-MS/MS fit-for purpose procedure for the quantification of Imatinib Mesylate in rat plasma can be used for pharmacokinetic studies in preclinical applications.
Bioanalytical method, Imatinib Mesylate, Rat plasma, Verapamil, Pharmacokinetics.
The drug, Imatinib an oral tyrosine kinase inhibitor, first line standard treatment in patients with Chronic Myeloid Leukemia (CML) and recurrent Gastro-Intestinal Stromal Tumor (GIST). Imatinib strongly improved therapy outcomes, however it was reported in many research articles that drug has large inter-individual variability in plasma concentration when standard dose was administered [1]. This also provides a research space for many researchers to work on a simple, sensitive, accurate and precise method development for quantitative and qualitative analysis of such type of drugs.
Additionally, the fitness for purpose of analytical methods is of major concern in drugs quantitative analysis. Over the past decade, the drug development paradigm has shifted to where we are looking for cost effective drug discovery and development therefore the research work have been directed towards small scale. Validation of each analytical method is crucial step in all pharmaceutical analytical laboratories, However, there is always been a lack of clarity in methodology in order to decide that the method can be considered as valid and reproducible [2,3]. Secondly accurate method helps in absolute bioavailability measurement, the rate and extent to which the drug is absorbed and becomes available at the site of measurement, subsequently producing therapeutic effect. Absolute bioavailability estimation is an important component to evaluate during New Drug Application (NDA) so as to assess the safety and efficacy of a drug product [4]. The Ultra-sensitive Liquid Chromatography coupled to tandem Mass Spectrometry (LC-MS/MS) application in bioavailability measurement is advancing with newer approaches like micro dose of either radiolabeled drug or stable isotope labelled drug [5].
In the present investigation we describe the method development and validation of a highly sensitive LC-MS/MS for the quantification of the anti-blood cancer drug Imatinib in rat plasma. The bioavailability (drug exposure) of a drug was calculated by measuring various pharmacokinetic parameters, non-compartmental pharmacokinetic was the approach utilized in the study.
Imatinib (4-[(4-methylpiperazin-1-yl) methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl) pyrimidin-2-yl] amino} phenyl) benzamide). Its investigational name was STI-571, is a tyrosine-kinase inhibitor used in the treatment of multiple cancers, the drug founds to be extremely useful in Philadelphia chromosome-positive (Ph+) Chronic Myelogenous Leukemia (CML) and significantly reduced mortality in CML patients. Imatinib was the first cancer therapy which to show the potential for such targeted action, therefore entirely changes the way CML was treated which ultimately gives the new ray of hope to these patients [6].
Imatinib Mesylate (IMT) was received as a generous gift sample from Neon Laboratories Limited Palghar. Verapamil used as internal standard was procured from Sigma-Aldrich. Dimethyl Sulfoxide (DMSO), Acetonitrile, Formic acid and all other pharmaceutical ingredients were purchased from Loba Chemie Mumbai, all were of analytical grade. Ultrapure water was obtained from a Milli-Q UF-Plus apparatus (Millipore Corp., Burlington, MA, USA).
Preparation of reagents and solutions
Body weights and dose calculations following oral administration of dose formulations:
Dose calculation and its estimation always require critical consideration about the difference in pharmacokinetics and pharmacodynamics among various under investigation species. Safe and effective drug dosing is important as the new laws and regulation been forwarded by different animal welfare agencies. There are several instances, wherein the initial dose of a particular drug is unavailable in a specific species. Therefore, choosing starting dose of such drugs for research, experiments, or clinical trials in animals and humans is a concern. Table 1 indicates calculations for dose subjected to respective total body weight following oral gavage administration to male Wistar rat.
| Treatment (Dose) |
Rat No. | Body weight (kg) | Target volume (mL) | Syringe weight (g) | Difference (g) |
Actual volume (mL) | Actual dose (mg/kg) F *TD/B |
Accuracy % | |
|---|---|---|---|---|---|---|---|---|---|
| A | B=A*V | Before dosing (C) | After dosing (D) | E=C-D | F=E/R | ||||
| Imatinib Mesylate (TD: 40 mg/kg) | Rj0765 | 0.263 | 2.63 | 9.37 | 6.50 | 2.87 | 2.87 | 43.7 | 109 |
| Rj0766 | 0.288 | 2.88 | 9.40 | 6.50 | 2.90 | 2.90 | 40.3 | 101 | |
| Rj0767 | 0.27 | 2.70 | 9.43 | 6.50 | 2.93 | 2.93 | 43.4 | 109 | |
| Imatinib Mesylate (TD: 40 mg/kg) | Rj0768 | 0.294 | 2.94 | 9.48 | 6.45 | 3.03 | 3.03 | 41.2 | 103 |
| Rj0769 | 0.285 | 2.85 | 9.37 | 6.45 | 2.92 | 2.92 | 41.0 | 102 | |
| Rj0770 | 0.294 | 2.94 | 9.54 | 6.45 | 3.09 | 3.09 | 42.0 | 105 | |
Table 1: Body weights and dose calculations before oral administration of dose.
Mobile phase A:
(0.1% Formic acid in 5 mM Ammonium Formate Solution)
Weighed 315.00 mg of ammonium formate and transferred it into a 1000 mL reagent bottle. Transferred 1000 mL of Milli-Q water into reagent bottle and added 1 ml of formic acid, solution mixed well and sonicated.
Mobile phase B:
(0.1% Formic acid solution in Acetonitrile)
Transferred 1000 mL of acetonitrile into 1000 mL reagent bottle, added 1 ml of formic acid and mixed well.
Preparation of 0.1% formic acid in Acetonitrile (percentage v/v)
0.2 mL of formic acid was added to 200 mL of acetonitrile in a reagent bottle and mixed well; this solution was used as precipitation solution.
Preparation of stock solution:
Imatinib weighed and appropriate volume of DMSO was added to achieve final concentration of 1 mg/mL. Dimethyl Sulfoxide (DMSO) stocks were mixed well and sonicated prior to use. In-vitro Stock of Verapamil (10 mM in DMSO) was used and diluted to 1 uM in 50 mL of acetonitrile.
Preparation of analyte working solution:
Working solutions for Calibration Curve (CC) standards (101 to 20000 ng/mL) of Imatinib were prepared in DMSO.
Internal standard working solution:
Internal Standard (IS) working standard solution was prepared by diluting 0.005 mL of IS stock solution to 50 mL with acetonitrile to provide a concentration of 1 μM. This solution mixed well and stored at 2°C to 8°C in a refrigerator until used.
Preparation of spiked CC standard and QC samples:
Calibration standards and quality control a sample of Imatinib was prepared by spiking the appropriate working solutions in blank matrices.
Sample preparation and HPLC conditions
Sample preparation was done by protein precipitation method. An aliquot (47.5 μL) of blank Matrix was spiked with 2.5 μL of analyte working solution.
CC and QC preparation:
20 μL of CC/QC/Study samples were aliquot into pre-labeled Eppendorf tubes and 10 μL of internal working standard solution was added. Samples were quenched with 250 μL of 0.1% formic acid in Acetonitrile and vortexes. All the samples were centrifuged at 14000 rpm for 5 minutes at 40°C. 150 μL of supernatant was transferred into inserts kept in 1 mL vials and caped with polyethylene plugs and analyzed in LC-MS/MS.
The extracts were analyzed using an LC-MS/MS system. HPLC components (all series 1100, Agilent Technologies, Palo Alto, CA, USA): binary pump; Pump A: 0.1% Formic acid in 5 mM Ammonium Formate Solution Pump B: 0.1% Formic acid in acetonitrile.
Table 2 gives HPLC conditions used in the process of drug estimation. A sciex API 4000 triple stage quadrupole mass spectrometer was used as detector. The HPLC systems were connected via a six-port column switching valve mounted on a step motor (Rheodyne, cotari, CA USA). The HPLCs, switching valve and the mass spectrometer conditions (Table 3) were controlled by the Analyst software (version 1.3.1., applied biosystems).
| Name of compound | Analyte | Internal standards |
|---|---|---|
| Imatinib | Verapamil | |
| Molecular weight of free compound (base/acid) | 493.60 | 454.6 |
| Diluent | DMSO | |
| Chromatographic conditions: | ||
| Mobile phase | Pump A: 0.1% Formic acid in 5 mM Ammonium Formate Solution Pump B: 0.1% Formic acid in acetonitrile |
|
| Gradient conditions | Isocratic Elution (Imatinib) Pump A : 40% Pump B: 60% |
|
| Analytical Column | PURITAS PNCN, 100 × 4.6 mm, 5 µ, CHROMACHEMIE | |
| Injection volume (µL) | 5 µL | |
| Flow rate (mL/min) | 0.8 | |
| Run time (min) | 5 | |
| Sample cooler temperature (°C) | 5 | |
| Column oven temperature (°C) | 40 | |
| Rinsing solution | Acetonitrile: Methanol: Milli Q water:DMSO-30:30:30:10 V/V | |
Table 2: HPLC conditions for bioanalytical method development.
| Instrument ID | API 4000 LC-MS/MS | |
|---|---|---|
| Mass parameters | Analyte Imatinib |
IS Verapamil |
| MRM transitions | 494.40 → 394.20 494.40 → 217.30 |
455.3 →165.10 |
| Resolution – Q1 | Unit | Unit |
| Resolution – Q3 | Unit | Unit |
| De-clustering Potential (DP) Volts | 100 | 86 |
| Entrance Potential (EP) | 10 | |
| Collision Energy (CE) Volts | 36 and 34 | 45 |
| Collision cell exit potential (CXP) Volts |
13 & 14 | 15 |
| Ionization / Polarity | Positive | |
| Dwell time (mille seconds) | 250 | |
| Ionization Source | ESI | |
| Collision gas (CAD) | 6 | |
| Curtain gas (CUR) | 25 | |
| GS1 | 40 | |
| GS2 | 60 | |
| Temperature | 500°C | |
Table 3: Mass spectrometric conditions for LC MS/MS method development.
Five microliters of the samples was injected on to a PURITAS PNCN, 100 × 4.6 mm, 5 μ, CHROMACHEMIE extraction column. The flow was 0.8 (mL/min), the mobile phase consisted of following gradient Pump A: 0.1% Formic acid in 5 mM Ammonium Formate Solution Pump B: 0.1% Formic acid in acetonitrile having Isocratic Elution (Imatinib) Pump A: 40% Pump B: 60%.
MS/MS analysis and quantification:
The triple stage quadrupole mass spectrometer and High Performance Liquid Chromatography (HPLC) system were coalesced by a turbo electrospray ion source. Nitrogen was the gas used for dissociation as a result of collision activation. The mass spectrometer was run in the positive MRM (multiple reaction monitoring) mode. The De-clustering Potential (DP) was set to -100 V. the interface were heated to 500°C, all other mass spectrometric conditions are shown in Table 3. The first quadrupole was set to select the [M+H]+ ions which was followed by monitoring of mass transitions: Imatinib (m/z 494.40 → 394.20), second mass transitions were monitored: Imatinib (m/z 494.40 → 217.20). Verapamil (IS, (m/z 455.30 → 165.10). Quantification was done by Imatinib- verapamil ratio calculations and comparison with non-weighted calibration curves.
Formulation analysis:
The formulations were diluted appropriately with DMSO and were analyzed using a known reference standard as comparison. Single point standardization method was adopted to quantify IMATINIB the formulations.
Method validation
Validation strategy:
Parameters determined for abbreviated validation included specificity, lower limit of quantification, linearity, intra-day accuracy and precision, and recovery of measurements.
Predefined acceptance criteria:
The predefined acceptance criteria were considered acceptable if precision (%CV) at each concentration was ≤ 10% for intraday and day-today variability. The accuracy compared with the nominal value had to be within ± 10% for both intra and day-to-day variability. The calibration curve had to have a correlation coefficient of r=0.9990
The specificity:
The chromatograms of three different batches of blank plasma with corresponding spiked plasma was evaluated by comparing it so as to establish specificity of developed method the to investigate the potential differences near the retention times of either the Imatinib or the internal standard Verapamil.
Lower limit of quantification:
The Limit of Detection (LOD) was defined as a signal-to-noise ratio of 3:1, the Lower Limit of Quantification (LLOQ) was determined as the lowest quantity consistently achieving accuracy ≤ ± 15% of the nominal concentration and a precision of ≤ 15% (Table 4).
| Intraday accuracy | Target volume | Rat plasma |
|---|---|---|
| LLOQ | 102.2% | |
| 1 ng/ml | 107.5% | |
| 10 ng/ml | 101.2% | |
| 100 ng/ml | 109.4% | |
| ULOQ | 96.7% | |
| Intraday precision | LLOQ | 6.4% |
| 1 ng/ml | 8.1% | |
| 10 ng/ml | 7.4% | |
| 100 ng/ml | 5.2% | |
| ULOQ | 3.8% |
Table 4: Intraday accuracy and intraday precision of IM quantification in rat plasma (LLOQ, lower limit of quantification; ULOQ, upper limit of quantification. LLOQ for rat plasma was 5.05 ng/mL).
Precision and accuracy
Linearity:
The standard samples were prepared to check the linearity of the method by the analysis of a series of standard samples with concentrations from 5.0 to 5000 ng/mL for Imatinib. The calibration curves were established through weighted linear least squares regression of the peak area ratios of the Imatinib to the verapamil (IS), obtained against corresponding concentrations.
Intra-day accuracy and precision:
Inter day precision and accuracy was determined based on quality control samples, which were determined by analyzing each of the three quality samples containing Imatinib (n=3) on the same day. Intraday samples were extracted and analyzed on three different days over a week (n=3). It was reported as coefficient of variance in percentage and accuracy was reported as a percentage of the nominal concentration.
Mass spectra and MS/MS spectra of the analytes were recorded after direct infusion of Imatinib into the electrospray source via syringe pump (KD Scientific, Holliston, MA USA). Study samples were aliquoted into pre-labelled Eppendorf tubes and 10 μL of internal working standard solution was added. Samples were quenched with 250 μL of 0.1% formic acid in Acetonitrile and vortexed. All the samples were centrifuged at 14000 rpm for 5 minutes at 40°C. 150 μL of supernatant was transferred into inserts kept in 1 mL vials and caped with polyethylene plugs subsequently delivered at a rate of 0.8 mL/Min.
In Figure 1, A gives structure for Imatinib mesylate and B shows the product ion scan spectra of Imatinib. The proton adduct of IM ([M+H]+, m/z 494.4) was the prominent Q1 ion. The fragment at m/ z=394.2 gave the fragment signal with the highest intensity thus the same transition was chosen for the quantification of IM. The most abundant product ion detected for the internal standard Verapamil was the ion at m/z 165.3. Based on this result, the transition m/z 455.5 to 165.3 was selected for the internal standard. In rat plasma, the lower limit of quantification was 5.05 ng/ml (Table 4) and the assay was linear from 5 to 5000 ng/ml (y=0.0315 x–0.0110, r=0.9991).
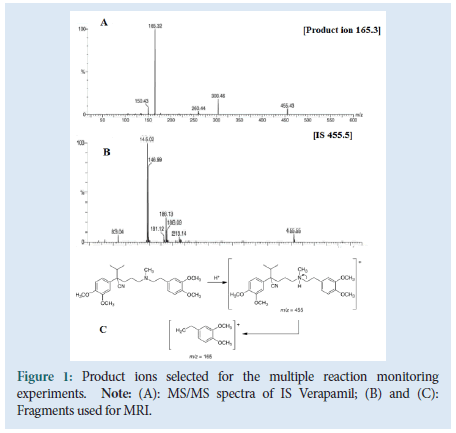
Figure 1:Product ions selected for the multiple reaction monitoring
experiments.
Note: (A): MS/MS spectra of IS Verapamil; (B) and (C):
Fragments used for MRI.
The absolute method recovery of IM after protein precipitation of rat blood was 93.4 ± 7.2% (n=3). The recovery of internal standard verapamil was 98.3 ± 6.2%. IM elution was with an average retention time of 3.21 ± 0.8 min and the internal standard verapamil with average retention time of 3.64 ± 0.5 m in. No obvious interferences from endogenous substances were observed.
Imatinib often referred as “magical bullet” as it has revolutionized the treatment of Chronic Myeloid Leukemia (CML) in 2001. Drug received FDA approval in May 2001. Lyndon, Druker, and the other inventers were awarded with many prestigious awards for “converting a fatal cancer into a manageable condition”. The development of a new therapeutic drug targeting cancer-specific molecules requires suitable methods for quantitative analysis. LC MS/MS is most widely acceptable and robust method of analysis for all newly discovered drugs [7,8].
Pharmacokinetic and bioavailability studies have been used by many researchers who had used LC-MS/MS (tandem mass spectrometry). Some of them have used an atmospheric pressure chemical ionization interface for detection and a semi-automated high throughput precipitation bioanalytic procedure for sample preparation. Others have used an electrospray ionization detection and non-automated precipitation followed by filtration. The method reported reached a LLOW of 4 ng/ mL IM, while Method was more sensitive with LLOQ of 1 ng/mL. Our LC-MS/MS assays fulfill all predefined acceptance with LLOQ of 5.05 ng/mL. This method can be utilized for quantification of IM directly as it was developed with the objective of fit-for purpose method [9-11].
Method application
The assay was successfully used for quantification of Imatinib for prepared formulations in the form Nano technology based drug delivery system called as Nanosponges (NSPs). The pharmacokinetic parameters were evaluated for prepared drug delivery system (Optimized NSPs) and marketed product [12]. The data processed for this preclinical study is shown in Tables 5A and 5B.
| Rat No. | Tmax | Cmax | AUClast | AUCinf | T1/2 | MRTinf |
|---|---|---|---|---|---|---|
| (h) | (ng/mL) | (ng.h/mL) | (ng.h/mL) | (h) | (h) | |
| Rja0765 | 1 | 6524 | 42832 | 44746 | 5.41 | 5.66 |
| Rja0766 | 1 | 8024 | 52339 | 57181 | 6.86 | 5.95 |
| Rja0767 | 1 | 9214 | 54075 | 59001 | 7.29 | 5.59 |
| N | 3 | 3 | 3 | 3 | 3 | 3 |
| Mean | 1 | 8021 | 49641 | 53629 | 6.51 | 5.70 |
| SD | NA | 1091 | 5042 | 6293 | 0.81 | 0.161 |
| CV% | NA | 0.139 | 0.099 | 0.118 | 0.123 | 0.027 |
Table 5A: Pharmacokinetic parameters of Imatinib following oral gavage administration (optimized NSPs).
| Rat No. | Tmax | Cmax | AUClast | AUCinf | T1/2 | MRTinf |
|---|---|---|---|---|---|---|
| (h) | (ng/mL) | (ng.h/mL) | (ng.h/mL) | (h) | (h) | |
| Rja0768 | 2 | 3910 | 31201 | 31231 | 4.77 | 6.12 |
| Rja0769 | 2 | 3530 | 33941 | 34349 | 3.62 | 5.91 |
| Rja0770 | 2 | 3250 | 32397 | 33614 | 5.02 | 6.22 |
| N | 3 | 3 | 3 | 3 | 3 | 3 |
| Mean | 2 | 3623 | 31989 | 32965 | 4.39 | 6.09 |
| SD | NA | 267 | 1204 | 1297 | 0.59 | 0.131 |
| CV% | NA | 0.0759 | 0.0345 | 0.0403 | 0.1364 | 0.0212 |
Table 5B: Marketed product in male Wistar rats (Dose: 40 mg/kg eq. to Imatinib).
The results were positive with respect to increased pharmacokinetic parameters for developed NSPs formulation which was optimized by quality by design approach.
A specific, sensitive and fit-for purpose LC-MS-MS method was developed and fully validated for the quantification of Imatinib in rat plasma. The major advantage of this validated method is its ready for use status along with simple, rapid and accurate estimation of model drug samples. Easy understanding for readers is possible for developed method as HPLC conditions is given in tabular form along with mass spectrometric system requirement which allows researcher to use it readily. It has excellent recovery and runtime of less than 4.0 minutes, which gives us high throughput analysis of number of samples. The method was successfully applied to pharmacokinetic study in rat plasma.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Pharmaceutical Methods received 3403 citations as per google scholar report