
Pharmaceutical Methods
Publishing Quality Research & Reviews

Pharmaceutical Methods
Publishing Quality Research & Reviews
Review Article - (2022) Volume 13, Issue 1
Received: Jan 03, 2022, Manuscript No. PHMETHODS-22-40587; Editor assigned: Jan 07, 2022, Pre QC No. PHMETHODS-22-40587 (PQ); Reviewed: Jan 21, 2022, QC No. PHMETHODS-22-40587; Revised: Jan 25, 2022, Manuscript No. PHMETHODS-22-40587 (R); Published: Feb 05, 2022, DOI: 10.35248/2229-4708.22.13.222
Methotrexate (MTX) is a potent drug for the treatment of various diseases globally amidst being a chemotherapeutic and immunosuppressant agent. However, hepatotoxicity induced by MTX could be life-threatening if left untreated. Folate supplementation is concurrently applied to reduce the adverse effects of MTX, albeit efficacy compromise. Therefore, there is the need to understand the process for the prevention and treatment strategies for MTX induced hepatotoxicity (MIH). In recent times, preliminary preclinical and clinical findings indicate the potential of natural phytobioactive compounds for MIH prevention and treatment. This mini review therefore summarizes proposed mechanisms of MIH and recent advances in the prevention and treatment prospects of natural phytobioactive compounds on MIH.
Phytobioactive compounds, Anti-inflammatory, Anti-oxidant, Methotrexate, Hepatotoxicity, Oxidative stress.
AICAR: 5-Aminoimidazole-4-Carboxamide- Ribonucleoside; GSH: Glutathione; Keap-1: Kelch-like Erythroid Cell-Derived Protein-1; IL-1β, IL-6 and IL-8: Interleukin-1β, -6 and -8; iNOS: Inducible Nitric Oxide Synthase; LPO: Lipid Peroxidation; MTXGlu: Methotrexate Polyglutamates; NF-Κb: Nuclear Factor Kappa B; Nrf2: Nuclear Factor-Erythroid-2 Related Factor 2; ROS: Reactive Oxygen Species; SOD: Superoxide Dismutase; TNF-α: Tumour Necrosis Factor Alpha.
Liver diseases are considered the most prevalent disorders worldwide and cover broad hepatic pathologies ranging from simple steatosis to chronic hepatitis, fibrosis, cirrhosis, hepatocellular carcinoma and acute liver failure [1-4]. This is worrisome as hepatic disorders generally result in disruption of the structural integrity of liver, thereby impeding vital functions in the maintenance and regulation of body homeostasis. One of such disorders causing havoc on human health is drug induced hepatotoxicity (DIH), this is defined as chemical-driven liver damage. This condition is well-documented to include some herbal medicines (administration of these agents in therapeutic windows or overdoses might damage liver) and chemicals- derived from industries and laboratories and industries and natural chemicals such as remedies of plants and microcystins might cause injury to liver. Currently, over 900 medications are culpably to be involved in inducing hepatic injury (termed as hepatoxins), which culminate in the major reason leading to drugs being unapproved or withdrawn [5-7].
Methotrexate (MTX) formerly termed as amethopterin, and a competitive dihydrofolate reductase inhibitor, has effectively been employed in the treatment of various rheumatological, oncological, dermatological, ectopically pregnancy, pancytopaenia and disorders of inflammation [8-11]. However, MTX is a well-known to induce hepatotoxicity in both humans [12] and animal models [13], albeit being the anchor drug for the treatment of rheumatoid arthritis and psoriasis [14] due to its cost effectiveness and potency. This in turn results in toxicity induced withdrawals in up to 30%-50% patients, thereby limiting its use as well as replacement with more expensive and toxic therapies [15,16] .Putatively, the life-threatening MTX induced hepatotoxicity (MIH) is caused by several mechanisms; nonetheless oxidative stress [17] and inflammation [18] have been well established. In view of this, MTX is concurrently prescribed with folic acid, nevertheless supplementation of folic acid and its derivatives are speculated to diminish the therapeutic efficacy of MTX, while their benefits are also controversial [19]. Due to these drawbacks, there has been an increasing search for novel strategies to reduce hepatotoxicity while optimising the efficacy of MTX [20]. Recently, supplementation of natural anti-oxidative and anti-inflammatory phytobioactive compounds has been documented to ameliorate MIH [21-23].
To the best of our knowledge no review has been documented on the potential of natural phytobioactive compounds for treatment and prevention of MIH. This mini review therefore summarizes proposed mechanisms of MIH, recent advances in the prevention and treatment prospects of natural phytobioactive compounds on MIH, as well as suggests development of therapies involving concurrent use of natural phytoconstituents with MTX.
Generally, DIH is manifested as a silent sub-clinical disorder or in association with several clinical conditions [1,24]. For effective alleviation of MIH, the exact mechanism should be clearly understood, however this is not the case [9], Nonetheless, recent investigations have hypothesised various mechanisms as playing vital roles in MIH, namely anti-oxidant defences coupled with increased oxidative stress, inhibition and activation of nuclear factor-erythroid-2 related factor 2-(Nrf2) anti-oxidant defence response (ARE)-nuclear factor kappa B (Nrf2-ARE-NF-κB) crosstalk, down-regulation of PPAR-γ as well as release of pro-inflammatory and apoptotic mediators [9,13,14,25,26]. Notwithstanding these results, understanding the detailed mechanism underlying MIH could help in attenuating the adverse hepatotoxic side effects of amethopterin therapy.
Oxidative stress, lipid peroxidation and MTX-induced hepatotoxicity
Several lines of evidences have documented the association between incidence of MIH and oxidative stress. These studies [14,27] suggest that MTX injured liver by negatively modulating mitochondrial machinery, wherein it resulted in uncontrolled production of reactive oxygen species (ROS) [14,28]. Consequently, this process leads to disruption of cellular macromolecules coupled with initiation of lipid peroxidation cascade and its concomitant cell death [29]. Additionally, it was established about two decades ago that MTX concentration is prolonged in intracellular via its conversion to polyglutamates in the liver.
Accordingly, this contributes to hepatotoxicity through cellular NADPH unavailability and glutathione reductase inhibition [30]. To date, this hypothesis has neither been corroborated nor refuted, thus further studies are needed for current understanding of this mechanism.
The MIH has been implicated in the depletion of mitochondrial enzymatic and non-enzymatic anti-oxidant defence systems through overproduction of ROS [31-33]. In recent times, emerging evidence demonstrates the involvement of Nrf2 Kelch-like erythroid cell-derived protein-1 (Keap-1) ARE-(Nrf2-Keap1-ARE) signalling pathway in regulating cellular resistance to oxidants [23,29]. As a class of basic-region leucine zipper (bZIP) protein, Nrf2 is well established to protect tissues against oxidative-induced injury via increased expression of cellular anti-oxidant defence proteins. Upon inducement of uncontrolled ROS production by MTX, the Nrf2 (generally suppressed in cytoplasm by Keap-1) [34] is activated. After transport of Nrf2 to nucleus, it binds to the ARE, which subsequently initiates transcription of anti-oxidative genes such as haem oxygenase 1 (HO-1) and NADPH quinone oxido-reductase-1 (NQO-1) [35]. Mechanistically, Nrf2 is activated via induction in two fronts, which are suppression and activation of Nrf2 under basal condition by inducers [36]. In this regard, there is an unmet need to identify natural phytobioactive compounds that can boost Nrf2 activation. However, with the emerging concept of Nrf2 functioning in double-edge sword manner [37,38], further investigations are required to unearth the exact impact of Nrf2 activation as well as actual role of its suppression in MIH.
Oxidative stress-induced lipid peroxidation is among the numerous mechanisms through which MTX causes liver injury. The uncontrolled ROS generated through MTX metabolism in liver attacks hepatocellular membranes resulting in lipid peroxidation, which potentially forms toxic lipid-derived aldehydes (LDAs), viz., acrolein, 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA) [38]. In accordance with this hypothesis, several authors have shown that MTX administration in rat models may induce evidential elevations of MDA and nitric oxide (NO) with concomitant decrease in the activities of catalase, glutathione (GSH) and superoxide dismutase (SOD) [9,18,39].
Despite the highly reactive NO culpability in the pathological process of hepatotoxicity induced by MTX [40], other authors [41] have posited that NO possesses hepatoprotective effect. Thus, NO acts a double- edged sword by reacting with superoxide radical, forming potent lipid peroxidation-inducing agent, peroxynitrite, while protecting the liver through inhibition of tumour necrosis factor-alpha (TNF-α) via NF-kB subunits modifications [41].
Role of inflammatory and apoptotic factors in MTX-induced hepatotoxicity
The involvement of inflammatory processes in hepatotoxicity induced by chemicals has been reported to occur through production of mediators that can cause liver damage or impede repair [42]. The release of inflammatory mediators during hepatic injury have been documented to include interleukin-1β (IL-1 β), IL-6, IL-8, NO and TNF-α [40,43,44] in animal models.
These cytokines and inflammatory mediators in concert with “master regulator of the inflammatory response (TNF-α) are capable of directly injuring liver [45]. Moreover, it has been established that inflammation is linked to apoptosis through TNF-α, which plays vital role in the homeostasis of liver [46]. Existing literature suggests that increased TNF-α expression result in the activation of apoptotic pathways (anti- apoptosis-NF-κB and pro-apoptosis-caspases) [47], which in turn cause MIH [43].
Further, it is speculated that proteins such as cyclooxygenases-2 and inducible NO synthase (iNOS, both regulated by NF-κB) generally influences the biological effect of TNF-α [48]. Also, it is possible MTX induced NF-κB signalling activation via 5-aminoimidazole-4-carboxamideribonucleoside (AICAR) [49] since its active and storage form, methotrexate polyglutamates (MTXGlu) can cause intracellular accumulation of AICAR through the inhibition of AICAR transformylase [50], thereby resulting in inflammation.
Moreover, TNF-α receptor-1 (TNFR-1) activation is assumed to be harbinger, wherein it is associated with cellular apoptosis initiation via activation of different types of caspases [51] (Figure 1). Among this family of protease enzymes is caspase-3, which often activates protease for cell death, thereby catalysing particular splitting of various important proteins in cells which consequently culminates in MTX-induced apoptosis [52]. Judging from preclinical studies, it seems plausible that inflammation and stress-related signalling pathways are the underlying mechanism of MIH. Thus, therapeutic strategies aimed at attenuating oxidative stress and its concomitant inflammation as well as enhancing cellular anti-oxidants can be explored to potentially prevent and treat MIH (Fig. 1).
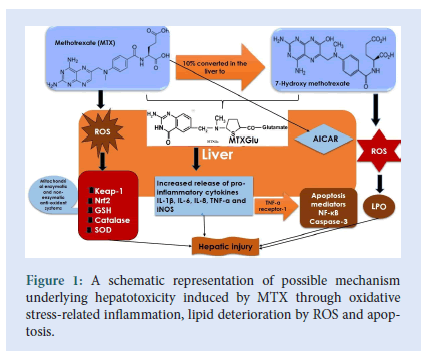
Figure 1:A schematic representation of possible mechanism underlying hepatotoxicity induced by MTX through oxidative stress-related inflammation, lipid deterioration by ROS and apoptosis.
Long-term use of MTX as first line treatment for chronic diseases such as rheumatoid arthritis and cancer normally result in increase in aminotransferases (also known as transaminitis) [53], which has recently received much recognition in clinical research [39]. Currently, prevention and treatment options for the aforementioned diseases are through concomitant use of folate supplementation (folic acid or folinic acid) and MTX to specifically minimize adverse effects including hepatotoxicity. Although, some studies have hinted that folate co-administration may not compromise MTX efficacy [54] but results of a post hoc analysis of two randomised control investigations suggested otherwise [55]. It is possible MIH is unrelated to folate antagonism unlike the other adverse effects such as anaemia, neutropaenia, stomatitis and oral ulcers [56]. Therefore, there is urgent need to search for novel preventive and treatment strategies. Current evidences indicate that supplementation of natural phytobioactive compounds with anti- oxidants properties might protect liver against MIH [25,27,33,52]. However, these preliminary findings should be confirmed in further studies through randomised clinical trials.
Natural products and its derived active constituents normally known as phytobioactive compounds have recently been explored in the treatment of several diseases [57-60]. In recent times, our exploits in phytocompounds research support the assertion that natural products have the potential to prevent and treat oxidative stress-related inflammation which underlie liver disorders [61,62]. Exemplary, Zhang and his colleagues established previously that natural bioactive phytocompounds from fruits and vegetables could ameliorate the incidence of several illnesses, while those possessing anti-oxidant properties could potentially reduce severe adverse effects of anti-tumour drugs in Chinese women in particular and global population as a whole. Invariably, there is growing optimism that these natural phytocompounds when supplemented with MTX could ameliorate its associated hepatotoxicity.
The prevention and treatment prospect of natural phytobioactive compounds on MIH for the past five years is summarized in Table 1. The preclinical and clinical evidences that we reviewed suggest that natural phytobioactive compounds were mainly comprised of polyphenols, saponins, isoquinoline alkaloids, flavonoid and phenols, which generally exhibited anti-oxidant, anti-inflammatory and anti-hepatotoxic properties against MIH (Table 1). Thus, the authors suggested that the understudied natural phytobioactive compounds exhibited promising hepatoprotective potential against hepatotoxicity of MTX. This in turn results in the maintenance of enzyme homeostasis in liver while providing increased anti-oxidant defence against oxidative-induced free radicals [63]. This is not surprising as several natural phytocompounds with anti-oxidant activities have been documented to protect liver against various hepatoxicans [6,22,64]. As postulated earlier by Brewer, the potency of wide range of anti-oxidant agents is proportional to the presence of hydroxyl (OH) groups on their aromatic rings [65]. This implies that phytocompounds with higher number of OH groups are likely to exert more effective anti-oxidant properties.
| Phytocompounds | Sources | Bioactivity | Research type | References |
|---|---|---|---|---|
| Chlorogenic acid | Hibiscus sabdariffa Solanum melongena Prunus persica Prunus domestica |
Anti-oxidant, anti-inflammatory, anti-apoptotic and anti-hepatotoxic |
Preclinical | 13 |
| Thymoquinone | Nigella sativa Oil | anti-oxidant and anti-hepatotoxic |
Clinical | 12 |
| Lauric acid | Virgin coconut oil (Cocos nucifera) | Anti-hepatotoxic and anti-lipid peroxidation | Preclinical | 25 |
| 18-β-glycyrrhetinic acid | Licorice root extract (Glycyrrhiza glabra) | Anti-oxidant, anti-inflammatory, anti-hepatotoxic and anti-apoptotic | Preclinical | 14 |
| Berberine | Coptis chinensis | Anti-oxidant, anti-inflammatory, anti-hepatotoxic, anti-apoptotic and anti-lipid peroxidation | Preclinical | 8 |
| Berberine | Coptidis rhizome (Rhizoma coptidis) | Anti-oxidant, anti-hepatotoxic and anti-lipid peroxidation | Preclinical | 68 |
| Ellagitannins (as punicalagins and free ellagic acid) | Pomegranate fruit extract (Punica granatum L.) | Anti-oxidant, anti-inflammatory, anti-hepatotoxic, anti-apoptotic and anti-lipid peroxidation | Preclinical | 23 |
| Resveratrol | Grapes,blueberries,raspberries, mulberries | Anti-hepatotoxic and anti-lipid peroxidation | Preclinical | 21 |
| Turmeric | Curcuma longa L., Zingiberaceae | Anti-inflammatory and anti-hepatotoxic | Preclinical | 33 |
| Gallic Acid | Green tea, gall nut, grapes, red wine , hops, oak bark etc | Anti-oxidant, anti-hepatotoxic and anti-lipid peroxidation | Preclinical | 69 |
Table 1: Prevention and treatment prospect of natural phytobioactive compounds on MTX induced hepatotoxicity (MIH).
Besides, natural phytocompounds demonstrate better anti-oxidative effect based on the diversity of their chemical structures and bioactivities compared to the currently available synthetic ones in the commercial functional foods and nutraceuticals [66]. In this regard the search for novel natural phytonutrients with anti-oxidant effect still remains a burgeoning field.
Due to growing body of evidences which suggest the overproduction of ROS and its associated oxidative stress-related inflammatory responses in playing crucial roles in the pathogenesis of various disorders [67-69], natural agents with both anti-inflammatory and anti-oxidant properties could be explored further to affirm their potency against MI (Table 1).
Up to now, there is no approved treatment strategy designed to cure MIH. Normally, folate is concurrently administered with MTX; however this approach has yielded inconclusive results. Current evidence hypothesises that MIH is initiated and progressed through oxidative stress-related inflammation, peroxidation and apoptosis. However, the process by which the metabolism of MTX induced overproduction of ROS is not clearly understood. Therefore, understanding the mechanisms of underlying MTX hepatotoxic effect would unearth the type of treatment strategies capable of preventing and treating MIH in humans. In view of this, pharmacological interventions of choice should be aimed at alleviating all the underlying mechanisms of MIH.
Preliminary preclinical and clinical findings show that natural phytobioactive compounds could prevent and treat MIH. This is promising, albeit several clinical trials needed to evaluate the actual effectiveness of this treatment option. In subsequent investigations, scientists should explore concurrent use of these natural phytonutrients with MTX and further assess their effects on MTX efficacy. Moreover, through nanotechnology techniques co-encapsulation of MTX and natural phytocompounds can be explored for the prevention and treatment of MIH.
Our sincere thanks go to authors whose published articles were used in this review.
All the authors report no potential conflicts of interest.
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [ [Indexed]
[Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed].
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
Pharmaceutical Methods received 3403 citations as per google scholar report