
Pharmaceutical Methods
Publishing Quality Research & Reviews

Pharmaceutical Methods
Publishing Quality Research & Reviews
Short Communication - (2023) Volume 14, Issue 3
Received: May 31, 2023, Manuscript No. PHMETHODS-23-100825; Editor assigned: Jun 02, 2023, Pre QC No. PHMETHODS-23-100825(PQ); Reviewed: Jun 16, 2023, QC No. PHMETHODS-23-100825; Revised: Jun 23, 2023, Manuscript No. PHMETHODS-23-100825(R); Published: Jun 30, 2023, DOI: 10.35248/2229-4708.23.14.256
For centuries humankind has been receiving methods to cure illnesses. During its development, medicine discovered several therapeutic tools. In ancient times herbal medicine started to find out what modern technologies could do, giving the base to it. For centuries, scientific development as well as seren-dipities pharmacognosy, pharmacology was brought to modern medicine. The passages got a complex asset of modern therapeutic tools passed not only by numerous fruitful discoveries but also by nefarious errors that severely hit human beings. History taught us what has been given us by living its consideration like a reprimand. Even if pharmacovigilance has now a robust scheme that can supply any articulated demand, will it be able to process now a pharmacological-biotechnological tool in the appropriate way? Is it ethical to push biotechnology forward and create a new chimaera?
excursiones privadas con guía de habla español en turquía
FPharmacology, Pharmacognosy, Pharmacovigilance, Drug development, Drug discovery, Biotechnology, Future prospective, Therapeutics tools.
The word pharmacognosy comes from two ancient Greek terms, φάρμακον pharmakon (drug or poison) and γνῶσις gnosis (knowledge). It studies natural drug derivation. As history teaches, since ancient times, the active ingredients contained within medicinal herbs allowed the cure of simple diseases. Yet, Pliny the Elder described in his treatise “Historia Naturalis”, the Papaver somniferum effect (Figure 1). He used the words "not only does it sleep, but still catching too much makes you die" in the twentieth book. Awhile, in the same book in paragraph 190, he says: "It is also useful that the juice of the decoction prepared by oil for head pains….” Similarly, Hippocrates of Kos in the Hippocraticum corpus, described willow bark, Salix as an analgesic and antipyretic remedy (Figure 2). In the same way as Pliny the Elder, the Greek physician Dioscorides and the Latin physician Galen identify the poppy pharmacognostic profile. Thus, drugs derived from plants guaranteed daily remedies for health problems since ancient times. During middle ages, the Persian physician Avicenna used opium, in the same way. Similarly, Paracelsus called the opium extract Laudan in 1522 describing its use. The Rever-end Edward Stone used 1757, the willow bark by Salix as an antimalarial in a small Oxfordshire village [1]. The history of health remedies development by pharmacognostic remedies proposes many models of drug development and several research perspectives.
Figure 1: Picture of Pliny the Elder
Figure 2: Morphology of Salix alba.
Through the centuries, extraction techniques improvement started pharmaceutical chemistry. Professor Jonas Anders Bruckner Felix Hoffman stabilised aspirin extraction in 1828, by preparing salycine from an aqueous willow bark Salix extract. Later, Felix Hoffmann synthesized aspirin which contains acetylsalicylic acids in 1897 [2]. Morphine discovery emerged by extracting lattice from the opium poppy plant Friedrich Sertürner. It happened in Germany [3]. Commercial productions began in 1827, by the pharmacy which became the Merck pharmaceutical company. During the late 19th century and throughout the twentieth century, leads for drug discovery became many so far. Discovering a druggable target follows a different strategy. Therapeutic leads also start with natural origin products. The steps to synthesise an active principle use combinatorial techniques as also rational drug design on molecular modelling.
New molecule design can increase pharmacodynamic profile by its Absorption Metabolism Distribution and Excretion (ADME) properties. The modification of phenanthrene nucleus morphine rings A B C gave birth to Fentanyl (Figure 3) [4]. Molecule modifications enhanced t1/2 increasing its effectiveness in prolonged treatment for chronic pain. However, an attempt at some molecule modification led to nefarious errors. Bayer synthesised and marketed Diacetylmorphine, “Heroin” in 1899 (Figure 4). Furthermore thalidomide, caused a disaster even if it is considered harmless in vivo. Its teratogenicity generates many cases of amyelia and phocomelia during two decades of market distribution.
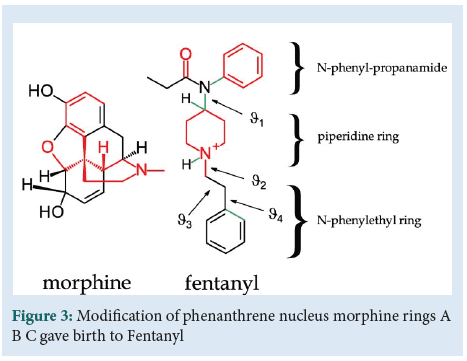
Figure 3: Modification of phenanthrene nucleus morphine rings A B C gave birth to Fentanyl

Figure 4: Bayer’s synthesized Heroine
The study of β-endorphins, similarly to the endocannabinoid system and cannabinoids, lead to the use of PEA Palmitoyl-eth- anol-amide to cure ulcerative colitis. Complex and non-modifiable structures such as the indolic alkaloids derived from Vinca rosea Catharanthus, Vincristine and Vinblastine treat tumours including Hodgkin's lymphoma. The studies on the active ingredient artemisinin which is derived from Artemisia annua yielded the Nobel prize award to the Chinese pharmacist Tu Youyou in 2015 (Figure 5). She used this remedy as an antimalarial. Certainly, ethnobotany and the use of the pharmacognostic derivate ion that leads to active principles left testimonies of what has been handed down over the centuries. Its cures started modern medicine through the testimonies of the ones who practised pharmacognostic remedies.
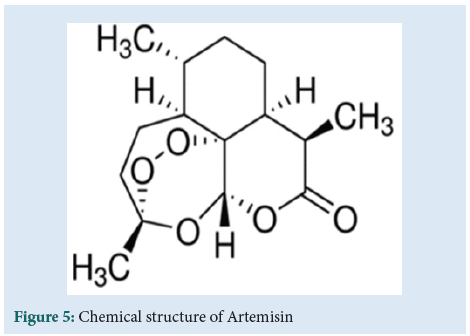
Figure 5: Chemical structure of Artemisin
Drug development goes through 3 pre-marketing stages. During phase 1, technical screening makes possible tests in vivo development. Its aims are:
• The affinity towards the receptor optimisation.
• The sites action optimisation increases molecule selectivity.
• Increase binding affinity by its binding site.
• Increase its efficacy and potency.
• Drug-receptor interaction development to assess pharmacodynamics profile.
• Obtain a safe drug.
• Estimate the minimal effective dose and the maximum toxic dose to improve step by step the pharmacokinetic profile Absorption, Distribution, Metabolism, and Execration (ADME).
Molecule development often starts from their natural mediators. It aims to match the natural and the endogenous precursors by the new ligand interaction by the receptor. Furthermore, personal pharmacognotic profiles enable the development of modern personalised therapy by biotechnological tools [5].
As in the fables of Aesop Ὁ μῦθος δηλοῖ ὅτι (ho mythos deloi oti: the fable teaches that….). What is the finding morality through the ages? How could the inherited historical examples be any framework for the development of actual biotechnologies? Could historical testimonies still lead to a new perspective in the drug discovery field? Indeed, the whole drug development process derives from testimonies left passed research. Future discovery doesn’t have any experience from passed historical discoveries. The basic preclinical test can assess basic toxicity as well as pharmacokinetic/pharmacodynamic profiles. Bioinformatic brings mathematical models into play. It can asset a precise toxicological profile as well as pharmaco-kinetic/pharmacodynamic on-body interaction. On the other hand, Pharmacovigilance should develop efficient shills to assess new types of side effects. The development of pharmacognosy using natural origin compounds started with the ancient Pliny the Elder, Hippocrates, Galen, Paracelsus, and Avicenna. It has shaped the development of modern medicine over the centuries, leaving space for fruitful discoveries like in the described cases E.g., Papaver somniferum, and Salix Artemisia annua.
The author declares no conflicts of interest. He conceived of and conceptualized the work, drafted the article, and critically revised the article. The author reviewed the literature and contributed to the outline and writing of the final manuscript.
No financial sources have been used to write this manuscript.
The authors have no conflicts of interest to declare.
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
Pharmaceutical Methods received 3403 citations as per google scholar report